Happy Holidays!Best wishes for Peace, Health, and Prosperity for you, your family, and your organization…
FDAs Final Rule on Laboratory Developed Tests (LDTs)
Update: FDA Rescinds Lab-Developed Test (LDT) Oversight Rule
On September 19, 2025, the FDA announced it will rescind its 2024 rule that extended medical device oversight to laboratory-developed tests (LDTs), officially ending the initiative to regulate LDTs as medical devices. The 2024 rule had amended the definition of “in vitro diagnostic products” in 21 CFR 809.3(a) to include those manufactured by laboratories. FDA has removed the words “including when the manufacturer of these products is a laboratory,” and reverted back to the original text.

On October 3, 2023, the FDA issued a proposed rule1 aimed at helping to ensure the safety and effectiveness of in vitro diagnostic products (IVDs) offered as LDTs, due to concerns that without FDA oversight false test results or false claims regarding the meaning of test results may lead to significant patient harm. See previous BMTA blog on “FDAs Proposed New Rule on Laboratory Developed Tests (LDTs)” for more details.
On April 29, 2024, FDA finalized the regulation on LDTs, addressing concerns raised during the public review and comment period. The final rule amends the FDA’s regulations by explicitly stating that IVDs, including those manufactured in a laboratory, are devices.2,3 Compliance with device requirements will help assure that IVDs, offered as LDTs, are safe and effective, allowing patients to have confidence in IVDs regardless of where they are manufactured.
In conjunction with this amendment, FDA finalized the phaseout policy of its general enforcement discretion approach for LDTs over the course of four years. After this phaseout, FDA will expect all IVDs (either made by laboratory or non-laboratory) to meet the same requirements. Specifically, this phaseout policy applies to IVDs that are manufactured and offered as LDTs by laboratories that are certified under CLIA and that meet the regulatory requirements under CLIA to perform high complexity testing and used within such laboratories. This includes IVDs that do not fall within FDA’s traditional understanding of an LDT because they are not designed, manufactured, and used within a single laboratory.
The general enforcement discretion approach timeline is:
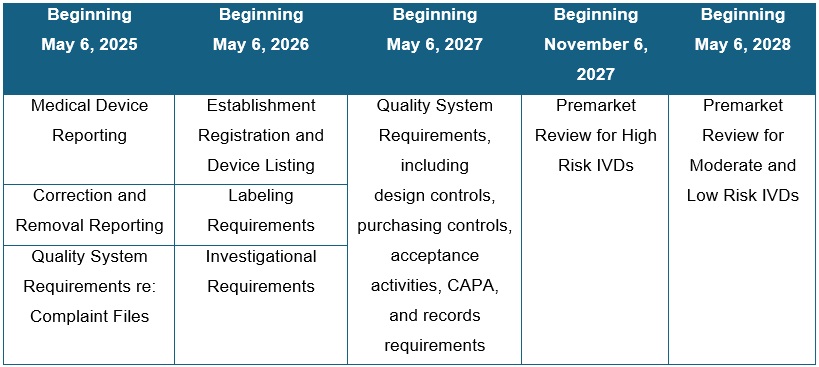
This phaseout policy does not apply to certain tests that were excluded from FDA’s general enforcement discretion approach, including tests that are intended as certain donor screening tests, tests intended for emergencies, direct-to-consumer tests without meaningful involvement by a licensed healthcare professional, and tests for use exclusively for public health surveillance.
In addition, FDA is adopting targeted enforcement discretion policies for several categories of IVDs manufactured by a laboratory in certain circumstances. For example, FDA intends to exercise enforcement discretion with respect to premarket review and most quality system requirements for:
- Currently marketed IVDs offered as LDTs that were first marketed before the LDT final rule was issued, as long as they are not modified after that date in regards to:
- Indications for useOperating principles (e.g., critical reaction components)Technology (e.g., addition of artificial intelligence)
- Performance or safety specifications (i.e., adverse changes)
- LDTs manufactured and performed by a laboratory integrated within a health care system to meet an unmet need of patients receiving care within the same health care system. Unmet need may be because:
- There is no FDA-authorized IVD for the disease or condition (e.g., IVD for a rare disease)
- There is an FDA-authorized IVD for the disease or condition, however,
- It is not indicated for the patient or does not sufficiently meet the patient’s needs (excluding potential performance improvements or lower cost)
- It is not available to the patient
FDA expects compliance with other device requirements for IVDs falling within these policies.
The new rule takes effect in July 2024. While some industry groups may challenge it in court, manufacturers of LDTs should start preparing to meet FDA regulations. This will ensure their tests comply with safety and effectiveness standards.

About Boston MedTech Advisors (BMTA):
Since 2004, BMTA’s multidisciplinary team of highly experienced consultants has supported more than 400 medical technologies and life sciences companies around the world to achieve their business goals. BMTA assists its clients to commercialize new products and services and increase their market adoption, by addressing their unique and inter-dependent regulatory, clinical, reimbursement, marketing, and business development requirements. BMTA offers valuable, ethical, result-oriented, professional, and cost-effective insights that recognize the multi-faceted aspects of today’s healthcare markets and the client’s unique business needs.
For more information, questions, or comments, contact us at info@bmtadvisors.com
Follow us on LinkedIn.
References:
- Proposed rule “Medical Devices; Laboratory Developed Tests” October 2023 https://www.federalregister.gov/documents/2023/10/03/2023-21662/medical-devices-laboratory-developed-tests
- FDA News Release: “FDA Takes Action Aimed at Helping to Ensure the Safety and Effectiveness of Laboratory Developed Tests” https://www.fda.gov/news-events/press-announcements/fda-takes-action-aimed-helping-ensure-safety-and-effectiveness-laboratory-developed-tests
- FDA Final Rule: Medical Devices; Laboratory Developed Tests https://www.govinfo.gov/content/pkg/FR-2024-05-06/pdf/2024-08935.pdf
Photo 313652465 © Justlight | Dreamstime.com

