Boston MedTech Advisors Blog, January 2024
Obtaining nationwide coverage for novel medical devices take an average of five years.1 In June 2023 CMS published a draft guideline for Transitional Coverage for Emerging Technologies (TCET). The new initiative is intended to provide expedited coverage for new technologies designated by the FDA as Breakthrough Devices [see BMTA blog for more information]2, even prior to the availability of clinical evidence typically expected to support coverage policies.3 Through the TCET, the CMS will finalize National Coverage Determinations (NCDs) for technologies selected for the TCET pathway within 6 months of FDA market authorization.

The proposed TCET program is based on experience gathered from the existing Coverage with Evidence Development (CED) pathway which enables Medicare to cover items and services that are furnished within approved clinical studies.4,5 Candidates for the TCET pathway include devices that are:
- Designated by the FDA as a Breakthrough Device.
- Not already the subject of an existing Medicare NCD.
- Determined to be within a Medicare benefit category.
- Not otherwise excluded from coverage through a law or a regulation.
TCET Process
The proposed TCET process consists of three stages: premarket, coverage under TCET, and transition to post-TCET coverage.
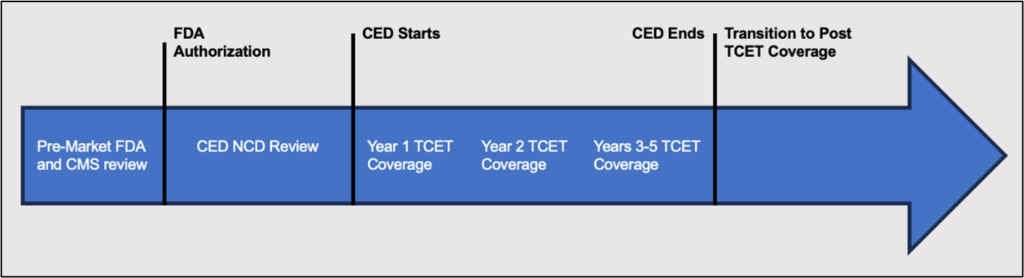
Pre-Market
- TCET pathway nomination. Submitted by the manufacturer of a the Breakthrough Device approximately 12 months prior to anticipated the FDA approval.
- Review and feedback. Initial meeting between CMS and the manufacturer to learn about the device.
- Benefit category review. CMS will deny applications for TCET for devices not covered through one or more Medicare’s benefit categories.
- Evidence review. CMS will conduct a literature review to assess coverage options.
- Approval of Evidence Development Plan (EDP). The CMS and the Agency for Healthcare Research and Quality (AHRQ) review and provide feedback on the proposed EDP within 30 business days following FDA approval of the technology. The manufacturer and CMS then have additional 60 days to adjust the EDP. Finalized EDP is submitted to CMS no later than 90 days after FDA approval.
Coverage Under the TCET Pathway
- Proposed TCET NCD. CMS will publish the proposed NCD once the device receives FDA and EDP approvals, and initiate a 30-day public comment period.
- Stakeholder meeting. CMS will solicit feedback from specialty societies and patient advocacy organizations.
- Finalizing the TCET. The new NCD will be published within 90 days following the release of the proposed NCD. The TCET may be in effect 3 to 5 years, though CMS will retain the right to reconsider the NCD at any time.
Transition to Post-TCET Coverage
- Updated evidence review. Conducted by CMS within 6 months of EDP review date. CMS and AHRQ will assess whether the evidence is sufficient and whether the conditions of coverage remain appropriate.
- NCD reconsideration. Based upon the updated evidence review, CMS may initiate an NCD reconsideration by posting a proposed decision with one of the following outcomes:
- An NCD without evidence development requirements.
- An NCD with continued evidence development requirements.
- A non-coverage NCD.
- Permitting local MAC discretion to determine coverage.
CMS is currently reviewing public comments on the proposed TCET pathway. Publication of the actual TCET program is expected during 2024.

About Boston MedTech Advisors (BMTA)
Since 2004, BMTA’s multidisciplinary team has supported over 400 medical technologies and life sciences companies to achieve their business goals. BMTA assists its clients to commercialize new products and services and increase their market adoption by addressing their unique and inter-dependent regulatory, clinical evidence, reimbursement, and marketing plans. BMTA offers results oriented insights that recognize the multi-faceted aspects of today’s healthcare markets and the client’s unique business needs.
For more information, questions, or comments, contact us at info@bmtadvisors.com
Follow us on LinkedIn.
References
- Waugh Ruggles S et al. The Need for Accelerated Medicare Coverage of Innovative Technologies: Impact on Patient Access and the Innovation Ecosystem, Health Management, Policy and Innovation. 2022;7(1). https://hmpi.org/2022/01/17/the-need-for-accelerated-medicare-coverage-of-innovative-technologies-impact-on-patient-access-and-the-innovation-ecosystem/#_edn1
- FDA Updates on the Breakthrough Devices Program: https://bmtadvisors.com/fda-updates-on-the-breakthrough-devices-program/
- Medicare Program; Transitional Coverage for Emerging Technologies. Federal Register; 88 FR 41633. https://www.federalregister.gov/documents/2023/06/27/2023-13544/medicare-program-transitional-coverage-for-emerging-technologies
- Program for Parallel Review of Medical Devices. Federal Register; 81 FR 73113. https://www.federalregister.gov/documents/2016/10/24/2016-25659/program-for-parallel-review-of-medical-devices
- Guidance for the Public, Industry, and CMS Staff: Coverage with Evidence Development. November 20, 2014. https://www.cms.gov/medicare-coverage-database/view/medicare-coverage-document.aspx?MCDId=27
- Farmer S et al. The Transitional Coverage for Emerging Technologies Pathway—Enhancing Innovation While Establishing Patient Safeguards. JAMA Health Forum. 2023;4(8):e232780. doi:10.1001/jamahealthforum.2023.2780
Photo 241576013 © Josepalbert13 Dreamstime.com

